|
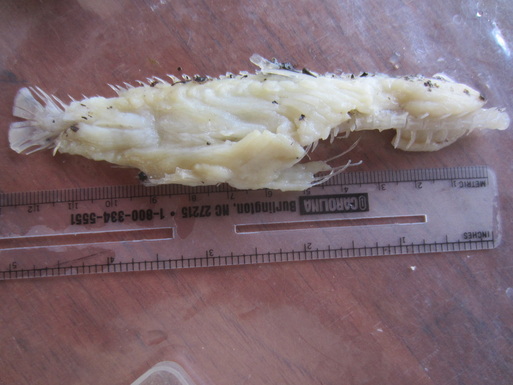
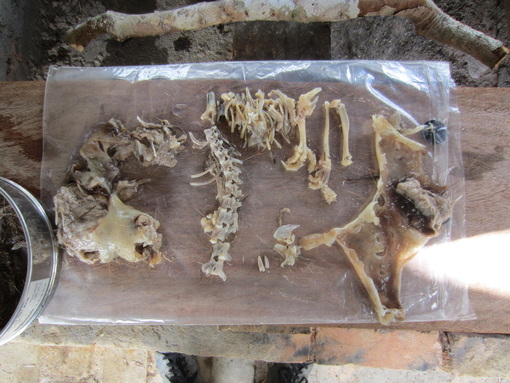
-- Adam Rosenblatt Post-doc  Photo Credit: Axel Schulz Photo Credit: Axel Schulz Catching caimans is not that complicated. First, you get in a boat at night, head out on the river, and use high-powered spotlights to find the animals. The light from the spotlights bounces off the tapetum lucidum (a layer of tissue within the retina that reflects light back into the eye, increasing the clarity of night vision), making the caiman’s eyes shine bright red from hundreds of meters away. Second, you drive the boat slowly towards the caiman, making as little noise as possible (crocodilians have good hearing). Third, a person at the bow of the boat uses a long pole to slip a metal snare around the neck of the caiman, and then tightens the snare by pulling on the rope it’s attached to or by yanking the pole upwards swiftly. Fourth, you hold on for dear life as the caiman thrashes around in the water, tiring itself out. Crocodilians tire relatively quickly (5 minutes or so) during bursts of intense physical activity because lactic acid, a natural by-product of cellular metabolism, builds up in their muscles and shuts them down. Human muscles also suffer from lactic acid build-up during exercise, but our muscles are able to get rid of it relatively quickly while crocodilians are stuck with it for longer periods of time because of their cold-blooded physiology. Fifth, you use a second snare to close the mouth and secure it, then you tape the mouth shut. Now you’re ready to safely handle the animal. During my time in Guyana my local counterparts and I caught 30 adult caiman this way, and to find out what they were eating we pumped their stomachs. Instead of explaining the whole stomach pumping process, you can watch this short video I made: https://www.youtube.com/watch?v=JCmogf-NQ2Y We ended up finding lots of interesting stuff in their stomachs, like snails, fish, seeds, and small mammals. Fish were by far the most frequently consumed type of prey, which means in the region I was in fishermen and caimans directly compete for food, at least during the dry season. The long-term challenge is to figure out how, despite this overlap, to maintain the quality of life for humans who depend on fish for food while also protecting the caiman population and the important roles caiman play in local food web and ecosystem dynamics. My goal is to go back to Guyana in the coming years to continue and expand upon the caiman research I began in February.
28 Comments
-- Adam Rosenblatt Post-doc Some research sites are really hard to get to. Back in 2007 I worked for 6 months in Shark Bay, Australia as a volunteer on Mike Heithaus’ long-running research project, and getting there was a schlep. I had to fly from Philly (where I lived at the time) to LA, then to Auckland, New Zealand, then to Perth, Australia, then take a bus 10 hours up the Australian west coast to a gas station in the middle of nowhere called “The Overlander,” then take another bus for an hour to the research site, Monkey Mia. By the end your bones ache and your eyes are weary with uncomfortable fits of sleep, but the beauty and possibility of such a remote place overtakes you and quickly you forget about the hardships of the journey. I recently did some research in another remote part of the world: Guyana. It’s a country few have heard of, tucked away on the northern coast of South America, dwarfed by its giant neighbor Brazil. Yet Guyana boasts vast tracts of untouched forests and savannahs, and some of the highest biodiversity on the planet. Guyana’s wilderness is impressive and dense, and it has remained largely undeveloped precisely because not many people know where it is or are aware of its natural splendor. I headed down there, to Guyana’s interior savannahs, to do some research on the largest crocodilian in the western hemisphere: the black caiman. To get there, I flew from New Haven to Miami where I picked up some supplies and equipment, then on to Port of Spain, Trinidad, then on to Georgetown, Guyana’s capital city. After a few days spent in Town (as the locals call it) to get my research permits, I hopped on a tiny little plane that flew me to a dirt airstrip near the Brazilian border where I was picked up by my local research collaborators in a red dust-covered pick-up truck. We drove 45 minutes over rain-scoured dirt roads and through a 3 mile long forest to the village of Yupukari, which sits on a hill above the Rupununi River and Awarikuru Lake. The Kanuku Mountains are located just to the south, and you can easily see them from Yupukari. The view is particularly beautiful in the evening when the mountains take on a purplish glow as the sun sets. The village is made up of about 1000 Macushi Amerindians, one of the indigenous groups in the region. It’s a fishing village in the dry season and a hunting village in the wet season, and I was there to figure out what black caimans eat and what ecological roles they play in the area. My base of operations was the lovely Caiman House eco-lodge, a popular place for tourists to hang their hats as they explore the amazing birds, fish, reptiles, and mammals of the region. When I arrived I unpacked my things in my breezy room, checked the integrity of the mosquito net hanging over my bed, and headed to the kitchen for a meal of fresh-caught fish, rice, and the spiciest chili peppers I’ve ever encountered. I was in paradise, or at least one version of it, and I was excited to see what surprises the surrounding waters held in store for me.
Next time: Caimans, and lots of them. -- Karin Burghardt, PhD Candidate For most people the trees losing their leaves for the year invokes a bit of sadness for the lost summer, however, I am breathing a sigh of relief. The end of the growing season and loss of leaves means a respite from fieldwork and a chance to reflect on how my experiment is shaping up (and how much more there is to be done). Last fall the lab helped me with an epic construction project to erect 28 raised beds at my field site and fill them with 20 tons of sand and 20 tons of field soil –accomplished by wheelbarrow (I know I owe them all my first born… but probably they would prefer cookies for life). In any case this past June I planted into the sandboxes mixtures of goldenrod genotypes known to express different plant defensive traits. The experiment also manipulates soil nutrients and herbivory. Over the next few years I will measure how plant defensive traits, herbivores, and nutrients influence plant and herbivore fitness and competition as well as trace nutrient cycling within the sandboxes. As a result, over the summer I spent a lot of time taking plant and nutrient measurements and playing Where’s Waldo with my grasshopper herbivores within the sandbox enclosures.
--Kassie Urban-Mead, Undergrad I’ve finished my first round of sampling for this summer’s research, and it feels great to be in the swing of fieldwork adventures. During last week’s sampling I stumbled across both a nesting duck and a spotted baby fawn: the former—a Blue-winged Teal—flapped away briskly while the latter flopped and stumbled endearingly into a nearby shrub. I’ve also alarmed countless Red-Winged Blackbirds, and while making my way back to the truck on Tuesday I noticed a mid-sized woodchuck peering at me from beneath a guardrail. My search, though, is for smaller critters. I’m not tracking Orthopterans like Rob and Bryan are, but I am solidly in the invertebrate camp! My senior research as a Yale undergraduate in Ecology and Evolutionary Biology is on the unmanaged bee pollinator communities in twelve old-fields across Connecticut. I’ve selected fields that are surrounded by a gradient of land-use types. These categories include forested, suburban development and roads, and conventional agriculture. This summer I’m sampling the bees (and recording the flowers I find them on) to see if there are community and interaction differences that can be traced to impacts from surrounding land-use. I’ve made my first round through each field, and the next set of collections will go into full swing when the sun comes out next week. All of this rain has been getting in the way (though it’s keeping those flowers growing!). It’s far too early to say anything for certain, but there are certainly distinct communities from field to field. I look forward to seeing how the species compositions shift once the flower resources are more similar—the goldenrod that dominates all of my fields hasn’t yet begun to flower (mostly Solidago rugosa and S. altissima), and the milkweed (Asclepias syriaca) and thistle (Carduus nutaans) are just beginning.
I’ll keep you updated, but it’s safe to say that the summer is off to a great start! I’m so lucky to work in some truly beautiful fields (and to have some great books on tape to listen to as I drive about between them). I’m rapidly becoming more and more adept at naming the wildflowers to genus and species, learning which long-tongued or short-tongued bees prefer, and knowing more of my bee IDs on sight. But most importantly? I think that my dexterity wielding insect nets in thick vegetation is preparing me for an alternate career as a badminton player! --Jennie Miller, PhD Candidate
Classes have ended and we’re ramping up for summer fieldwork here in the Schmitz Lab. Five of the Schmitzers will be working in the field, carrying out innovative projects with lizards, spiders, grasshoppers and pollinators in Greece, Connecticut and Vermont. Colin departed three weeks ago for the islands of Greece, where he’s collecting preliminary data on lizard morphology in the presence of predators and rock wall refugia. Colin will be catching lizards and measuring every diagnostic metric he can think of to motivate his upcoming dissertation experiments. He left for Greece with some pretty cool field equipment, including snake tongs and a lizard bite balance. Hope the lizards are biting, Colin! Karin will be starting the sandbox experiment of her dissertation, which will run for the next few years. In June, she’ll transplant Solidago altissima plants from our greenhouse into cages in the fields of nearby Wallingford, CT. She’ll catch grasshoppers and stock the cages to examine their effects on the plants. Bryan will be studying how local adaptation in grasshoppers affects ecosystem response to climate warming. He’ll be comparing how grasshoppers from Connecticut (which likely adapted to handle warm temperatures) and grasshoppers from Vermont (expected to handle cooler temperatures) affect plant communities. Bryan plans to carry out a transplant experiment in which he’ll move CT grasshoppers to VT and likewise VT grasshoppers to CT and then examine differences in how the grasshoppers consume plants in field cages subjected to warmed conditions (simulating climate change). Bryan expects that the cool-adapted VT population of grasshoppers will be more phenotypically plastic in its response to warming. Rob has been prepping for fieldwork this summer by working in the lab, exposing Solidago plants to nitrogen and examining how the addition of nutrients to the soil impacts the rate of nitrogen cycling. He’ll be carrying out complimentary field experiments at the Yale Myers Forest, in which he’ll expose caged, old-field plots to different aboveground and belowground community compositions by altering the presence of herbivore grasshoppers, carnivorous spiders and microbial grazing springtails. Our undergrad Kassie will spend her first summer with the Schmitz Lab up at the Yale Myers Forest. She’ll be studying native pollinator community and plant-insect interaction webs across an anthropogenic impact gradient. Kassie is a big fan of native bees, which she believes are an important component of ecosystem health and are increasingly important as managed bee populations are in decline (especially the European honey bee Apis mellifera). This summer Kassie will spend her days watching bees, searching for correlative ecological, life history and landscape clues to the most important factors that support or disrupt pollinator communities. Good luck Kassie! Meanwhile, back in Greeley lab, Anne, Kevin and I will make ground-shaking progress in our spatial analyses of animal movement, distribution and predation patterns while sunbathing in the botanical garden to keep up with our labmates’ tans. Here’s to a fantastic summer! --Kevin McLean, PhD Candidate I looked at my calendar this morning and realized that it has now been over 7 weeks since I returned from my field season in Panama. Seeing as I was only gone for 12 weeks, I suppose it is about time that I stop telling people I am still “adjusting to being back.” With that out of the way, on to the fieldwork update! I departed for Panama in mid-December to finish up the necessary training and do a bit of testing and tinkering for my dissertation research. A portion of my project will involve monitoring arboreal (tree-dwelling) mammals in the rainforest canopy using motion-sensitive cameras, or “camera traps.” While I’ve spent a fair amount of time learning how to use camera traps on the ground, setting them up 6-10 stories above the forest floor was a whole new challenge. First on the agenda was learning how to actually get into the trees. I spent three weeks at the Institute for Tropical Ecology and Conservation (ITEC) taking a tree climbing course, which I sometimes refer to as “Canopy Access Techniques for Research” to sound unbelievably fancy. While three weeks may sound excessive, one of the many goals I set for myself while working on my dissertation was to not die, and my instructor Joe Maher played integral role in giving me the training necessary to succeed in that regard. The next step was figuring out how to mount the cameras in the trees. I had a fair amount of help with this from Dr. Tremaine Gregory, who was kind enough to share the designs she used for her work in Peru. I put together a set of six mounts using a couple different designs that allowed me to monitor animals on pretty much any branch size or angle. Once I got some initial troubleshooting out of the way, I was able to get some pretty great photos of the arboreal wildlife in Bocas del Toro. Some of the species that crossed the cameras included capuchin monkeys, green iguanas, woolly opossums, and climbing rats. After about a month in Bocas I traveled to the Canal Zone to set up my cameras in the forests in the Barro Colorado Island Nature Monument. Dr. Stefan Schnitzer, the primary investigator for the Liana Ecology Project was kind enough to allow me to set up cameras on his research plots on the Gigante Peninsula. I chose two trees on Gigante peninsula, one in a plot in which all the lianas had been removed, and another in a control plot. Once again it took a bit of fiddling to get things running smoothly, but after a several solid weeks I managed to get photos of more capuchins and woolly opossums, as well as some coatimundis, kinkajous, squirrels, and toucans.
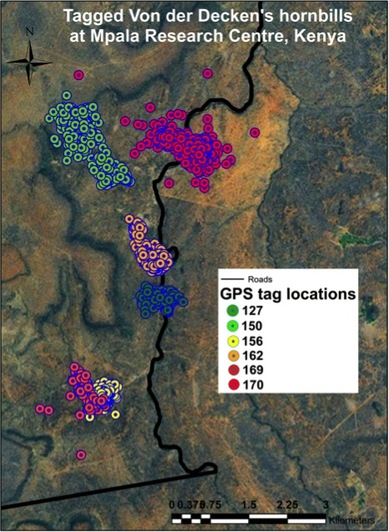
I thankfully made it back to New Haven in one piece, where I will spend my time going through photos and working on the other pieces of my dissertation until I return to Panama January 2014.  Why do species occur where they do? How do we accurately predict their occurrence? These questions have inspired naturalists and scientists for many years. Accurate species occurrence information can be used to select protected areas, identify movement corridors, and develop successful management practices. The research I conduct at Mpala Research Centre in Laikipia, Kenya investigates how environmental conditions and spatial scale influence species occurrence patterns. Both humans and animals are known to select resources at different spatial scales. For example, when choosing a city in which to live, I might consider weather patterns, local amenities, and job availability. When purchasing a home, I would want to know a house’s square footage, yard size, and safety. Animals respond to environmental conditions at different scales in similar ways. When selecting a home range, a bird might prioritize food resources, water availability, and nearby competitors. When selecting a nest site within a home range, ease of entry and shelter from predators may be more important. These habitat selection processes, combined across many individuals, determine a species’ local distribution. The first step in studying scale-dependent occurrence patterns is to measure the scales where habitat selection takes place. One method uses movements made by individuals over long time periods. Places where animals move short distances and turn in many directions likely contain important resources like food; places where animals move long distances over short time periods might be dangerous or contain less food or water. Recent technological developments make it possible for very small GPS tags (less than 20 grams) to collect a precise GPS location every 20 minutes, and run for months on solar-powered batteries. During August – October 2012, my team attached GPS tags (built by the University of Konstanz, Germany) to six adult Von der Decken’s hornbills at Mpala. One tagged hornbill often flies by the research station and dining area, showing off his GPS “backpack.” Movement data are collected by placing weatherproofed antennas, receivers, and memory units near where tagged birds roost at night (search www.movebank.org to see where tagged birds spend their time at Mpala Research Centre). After several months, we will collect enough movement data to identify important spatial scales for von Der Decken’s hornbills. Once these critical features are measured, we will build models that incorporate data on environmental conditions to understand how hornbills select habitat and resources across spatial scales. Measuring the scales that are important to different species will also help us understand how species coexist by splitting environmental resources. Together, a fuller understanding of scale dynamics and the detailed information collected by GPS tags will move us closer to understanding and predicting species occurrence patterns.
--Jennie Miller, PhD Candidate
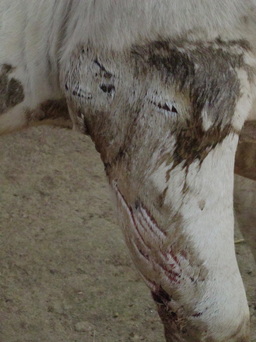
Last winter in November 2011, I headed to India's Kanha Tiger Reserve to survey livestock attacked by tigers and leopards. What I discovered upon arrival shocked and compelled me: livestock die every day, yet local people are surprisingly tolerant of the large cats that kill their animals. The livestock compensation program within the boundaries of Kanha appears to be successfully abating tempers and minimizing retaliation against tigers and leopards. Three seasons, 484 dead livestock, 115 owner interviews and 152 stinky cat scat samples later, I returned to Yale in mid-October to analyze my data. To read more about my last year of fieldwork and the gist of my research, check out my guest blog on Scientists Without Borders and my just-released article in Yale's Sage Magazine. |
Archives
November 2019
Categories
All
|
























 RSS Feed
RSS Feed
